Atrial fibrillation (AF) is the most common type of irregular heart rhythm, affecting more than 2 million Americans. The incidence of AF increases with age, afflicting about 4 percent of the population older than 60 and approximately 10 percent of people older than 80. The actual prevalence may be much higher since many people with AF are asymptomatic, and patients who have paroxysmal (intermittent) AF may be undersampled. As the North American population ages, the prevalence of AF will continue to rise. It is predicted that the number of Americans diagnosed with AF will grow to more than 10 million by the year 2050.
Although atrial fibrillation is often considered a harmless arrhythmia, it is associated with serious illness and death because of its effects:
- Palpitations, resulting in patient discomfort and anxiety
- The heart’s inefficiency in circulating blood, which may lead to cardiomyopathy (enlargement of the heart) and heart failure
- Lack of blood flow in the left atrium, which increases the risk of thromboembolism and stroke. It has been estimated that AF increases the risk of stroke by three to five times. Of all acute stroke patients, 20-30% are found to be in atrial fibrillation.
Physiological Basis of AF
Atrial fibrillation is characterized by the irregular activation of the atria (the upper chambers of the heart) and an irregular response by the ventricles (the lower heart chambers).
In a person with a normal heart rhythm, electrical signals from the sinoatrial (SA) node cause the atria to contract. This electrical impulse travels from the SA node (located in the right atrium) to the left atrium and down the interatrial septum to the atrioventricular (AV) node. At the AV node, the electrical impulse splits off to the left and right bundle branch. This is where the electrical stimulation causes the ventricles to contract and force blood out of the heart to the lungs and body. This organized, synchronous electrical impulse is called normal sinus rhythm.
There are several systems of classifying atrial fibrillation, including the historic paroxysmal (intermittent) and chronic AF. The classification system published jointly by the American Heart Association, American College of Cardiology and Heart Rhythm Society is the most widely used. This system defines AF as paroxysmal, persistent or permanent. When a patient has had two or more episodes, AF is considered recurrent. If recurrent AF terminates by itself, it is designated paroxysmal, but if it does not, it is termed persistent. Termination of AF by medication or electrical cardioversion before the expected spontaneous termination does not change the designation paroxysmal. Permanent AF includes cases of long-standing disease (longer than one year), in which cardioversion has not been indicated or has failed to convert the arrhythmia. This terminology applies to episodes of AF that last more than 30 seconds and that are unrelated to a reversible cause.
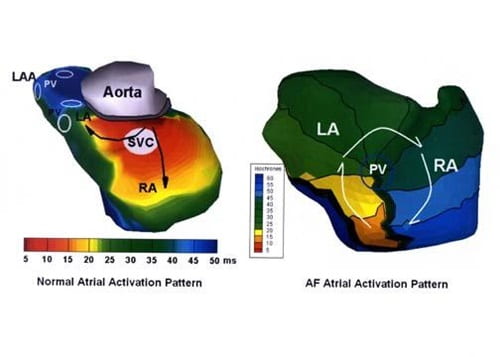
Recently, there has been emphasis placed on the role of the pulmonary veins in atrial fibrillation. Paroxysmal AF often originates in the pulmonary veins. Mapping studies suggest that the trigger for initiating and maintaining AF is located in the pulmonary veins slightly more than 50% of the time in the paroxysmal and persistent AF groups. Meanwhile, our data indicate many patients have multiple potential drivers of AF. Although some data suggest that pulmonary vein isolation is effective in 70-80% percent of paroxysmal AF cases, it is clear that pulmonary veins are not involved 20-30% of the time. And, in persistent AF, pulmonary vein isolation alone has a much lower success rate. Currently, diagnostic technologies rarely allow for a preoperative determination of the source of AF.
History of Surgical Treatment of Atrial Fibrillation
Because of the limitations of medical therapy for atrial fibrillation, there was a strong interest in the non-pharmacological treatment of the disease in the 1980s and 1990s, which led to the development of various catheter-based and surgical techniques. Although many of these techniques were not successful in addressing all of the effects of AF, they helped physicians gain fundamental knowledge of the mechanism of AF and laid a foundation for the development of the Cox-Maze procedure.
Three surgical procedures developed in the early 1980s – the left atrial isolation procedure, catheter ablation of the AV node-His bundle complex and corridor procedure – attempted to isolate and confine AF to a certain region of the atria, preventing it from spreading its effects to the ventricles. None of these procedures were targeted to cure the AF itself.
In 1985, James Cox, M.D., and his group at Washington University School of Medicine and Barnes-Jewish Hospital described, for the first time, a series of experiments that attempted to cure AF in a canine model. After a number of experiments, it was found that a single long incision across both atria and down into the septum cured AF. This “atrial transsection” procedure prevented the induction and maintenance of atrial fibrillation or atrial flutter in every canine treated.
Unfortunately, this procedure was effective but not curative in its clinical application. It soon became apparent that the surgical cure of AF would require a more complete understanding of the fundamental electrophysiological mechanisms of the illness.
Development of the Cox-Maze Procedure
Extensive experimental investigation under the leadership of Dr. Cox led to the introduction of the Maze procedure in 1987. The Cox-Maze procedure was designed to interrupt any and all macro-reentrant circuits that might potentially develop in the atria, thereby eliminating the chaotic web of electric impulses that spread throughout the atria and preventing flutter or fibrillation. Essentially, surgeons make small, strategically placed incisions in the atria. The incisions generate scar tissues that serve as barriers, trapping abnormal electric signals in a “maze” of barricades. Only one path remains intact, guiding impulses to their correct destination. In contrast to the earlier procedures, the Maze procedure successfully restored both atrioventricular synchrony and a regular heartbeat, thus significantly decreasing the risk of thromboembolism and stroke. It also allowed all of the atrial myocardium (middle muscular layer of the heart wall) to be activated, resulting in preservation of atrial transport function in most patients.
During the operation, the pulmonary veins also are completely isolated. This has proven to be fortuitous as the importance of the pulmonary veins in the initiation of AF has become more appreciated in recent years. The left atrial appendage (LAA) is either sewn closed or completely removed during surgery. This is performed because the LAA is a common source of clot development and serves to greatly reduce the risk of stroke in this population.
The original Maze procedure performed in the 1980s was modified because of the increased incidence of pacemaker implantation. However, the second Maze procedure proved to be very difficult technically to perform. It was therefore modified again to become the Maze-III procedure, also known as the Cox-Maze III procedure today.
The Cox-Maze procedure became the gold standard for the surgical treatment of AF. In a long-term study of patients who had the Cox-Maze procedure at Barnes-Jewish Hospital, 97 percent of patients at late follow-up were free of the disease. Although other institutions have experienced good results, our group has achieved the best long-term results in the world. Our team also has taught surgeons from around the world how to perform this operation.
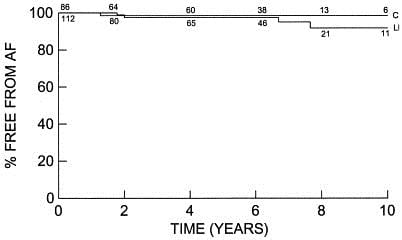
Kaplan-Meier survival analysis of freedom from recurrent AF. The numbers on each line indicate the number of patients at risk. There was no difference in the long-term estimate of freedom from AF between the lone maze group (L) and the concomitant group (C; P = .64). (In the LM group, the freedom from AF was 92% at 10 years. In the CM group, the freedom from AF was 97% at 10 years.)
Reprinted from The Journal of Thoracic and Cardiovascular Surgery, Volume 126, Issue 6, December 2003, Page 1825. Copyright 2007, with permission from Elsevier.
Modified Cox-Maze Procedure Using Bipolar Radiofrequency Energy
Despite its proven effectiveness, the Cox-Maze III procedure did not gain widespread acceptance. Few cardiac surgeons were willing to add the procedure to a coronary revascularization or valve procedure because of its complexity and technical difficulty. In an attempt to simplify the operation and make it more accessible to the average surgeon, groups around the world have replaced the incision of the traditional cut-and-sew Cox-Maze III procedure with linear lines of ablation. These linear lines of ablation are created using a variety of energy sources including radiofrequency energy, microwave, cryoablation, laser and high-frequency ultrasound.
The development of these new ablation technologies has revolutionized the surgical treatment of AF by taking a technically difficult and time-consuming operation and making it easy for all cardiac surgeons to perform. Today, most patients with chronic AF undergoing open heart surgery are offered a modified Maze procedure as part of the operation. Another advantage of ablation technologies is the promise of development of less-invasive procedures for AF via a small incision or port.
At Washington University, bipolar radiofrequency energy has been used successfully to replace most of the surgical incisions of the Cox-Maze III procedure. The current procedure incorporates lesions identical to those of the Cox-Maze III procedure, and has been named the Cox-Maze IV procedure. Clinical data have shown that this modified operation has significantly shortened the operative time while maintaining the high success rate of the traditional cut-and-sew Cox-Maze III procedure.
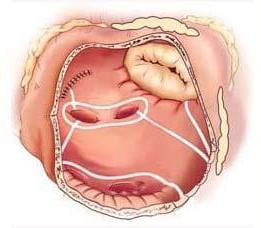

Right atrial lesions of the Cox-Maze IV procedure.